
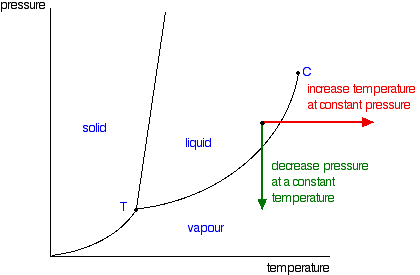

Pressure on the surface of water tends to keep the water molecules contained. Pressure Affects the Boiling Point When atmospheric pressure increases, the boiling point becomes higher, and when atmospheric pressure decreases (as it does when elevation increases), the boiling point becomes lower. Does water boil at higher temperatures at higher pressures explain? What is the effect of pressure on boiling point Class 9? As the water boils, heat is lost due to the heat of vaporization of water, which is 40.88 kJ/mol. When the pressure above a liquid is reduced, the vapor pressure needed to induce boiling is also reduced, and the boiling point of the liquid decreases.


 0 kommentar(er)
0 kommentar(er)
